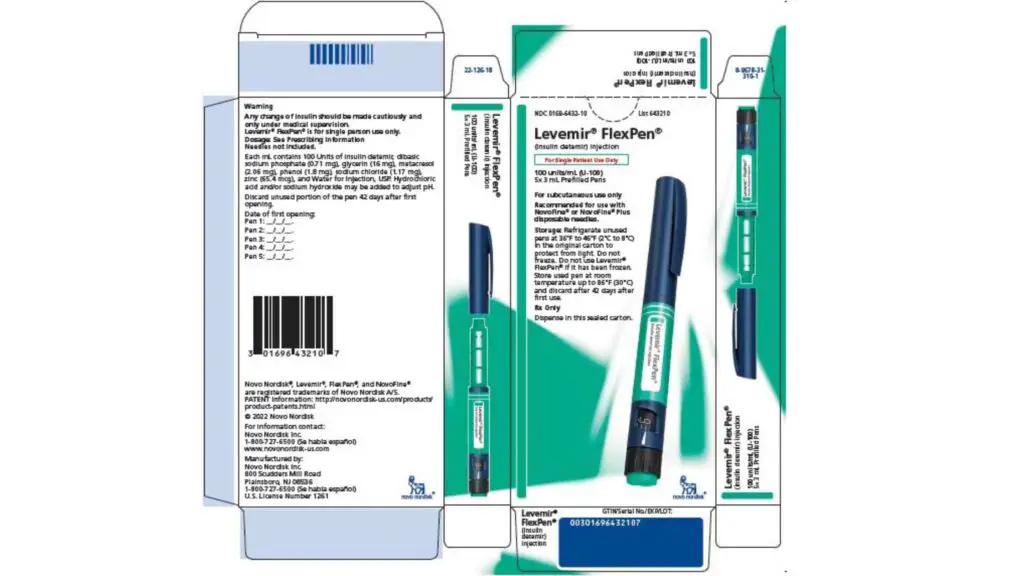
Is Levemir Flextouch discontinued? Beginning in early February 2023, Levemir FlexTouch will no longer be available. It will be replaced by a slightly different prefilled pen called the Levemir FlexPen. When customers pick up their pen from the drugstore, they will note that it appears a little extra if they are currently on Levemir.
Levemir is a long-acting insulin that can be used once or twice a day by adults and children with diabetes to manage high blood sugar levels. Levemir is an artificial insulin that is used to treat diabetes mellitus in both adults and kids. Levemir is ineffective for those with diabetic ketoacidosis (which increases ketones in the blood or urine).
Novo Nordisk is no longer producing Levemir Flextouch pens. But the company is still selling them until supplies run out. According to the business, Levemir Flexpens are expected to go on sale in early February 2023.
DiscontinuedNews is impartial and independent, and every day, we create distinctive, world-class programs, news, and content that inform, educate and entertain millions of people worldwide.
What happened to Levemir flextouch?

Insulin detemir [rDNA origin] injection, known as Levemir FlexTouch, received FDA approval in December 2014. It was the most recent insulin delivery pen from Novo Nordisk. An analog of long-acting human insulin is present in it. It is designed to assist diabetic adults and kids in maintaining better glycemic control. Levemir FlexTouch is not advised for diabetic ketoacidosis. Instead, injectable rapid-acting or short-acting insulin should be utilized, according to the approval’s restriction.
The FDA initially approved Levemir in 2005. Later, it was supplied as a preloaded pen device called the Levemir FlexPen or as a 10-mL multidose vial. Levemir FlexPen is projected to be discontinued in place of the Levemir FlexTouch in 2014. In the UK, Canada, Denmark, and Japan, Levemir FlexTouch was introduced.
Novo Nordisk voluntarily recalled 1,468 product samples in May 2021. The recall was for the customer levels of Levemir, Tresiba, Fiasp, Novolog, and Xultophy. It’s because they were kept at temperatures below the recommended range. Thus, these products are being recalled. Only product samples are affected by this announcement. The items that have been widely distributed to pharmacies or mail-order businesses are unaffected by this recall.
The cartridges and pen injectors may become ineffective and suffer damage if product samples are kept at temperatures below 32 °F. Customers risk not getting the correct medicine dosage if they take a product from a poorly stored vial, cartridge, or pen injector. This could cause hyperglycemia or hypoglycemia, which could have adverse health effects ranging from minor to life-threatening. No complaints of severe side effects or injuries associated with this recall for Novo Nordisk.
These medications are delivered in cartons. It comes with a vial, a pen injector (FlexPen or FlexTouch), or a cartridge to reduce blood sugar levels in people with diabetes (PenFill).
What is a replacement for Levemir?
There have been significant supply issues for Novo Nordisk products. This is a result of the extremely high patient demand. To increase production capacity for the patients they treat, the company is doing both urgent and long-term things. Novo Nordisk will switch from FlexTouch to its original FlexPen device for Levemir to assist with existing supply problems. This will reduce the pressure on the production lines.
Rising patient demand throughout the entire Novo Nordisk line of products is the reason for this change. Levemir patients can continue getting their medication because the FDA has already approved Levemir FlexPen. Additionally, it helps Novo Nordisk better serve the needs of the patients they support.
Beginning in early February 2023, the Levemir FlexPen will be offered for purchase. Levemir FlexTouch will continue to be supplied until stock runs out, which will differ from location to location across the United States.
Patients must accept updated Instructions because FlexTouch and FlexPen have various features and functions. The changeover from FlexTouch to FlexPen will not alter the active medication. Thus, it will not impact Levemir’s effectiveness or safety profile.
Patients who manage their diabetes should talk to their doctors about dosing options. Patients who currently use Levemir FlexTouch and require more than the Levemir FlexPen’s limit of 60 units per injection are included.
Why Levemir flextouch out of stock?
Patients with refills can keep getting their Levemir FlexTouch as long as stocks remain. Some pharmacists may need to inform their practitioners about the switch after the FlexTouch supply runs out. This is necessary to ensure that the Levemir FlexPen is approved. They should take proactive steps to ensure that the Levemir patients also have an updated prescription for Levemir FlexPen to minimize disruptions.
Also, patients, pharmacies, and distributors are not required to return this medicine if they have it on hand. This is because the change is not the outcome of any safety or performance problems with Levemir FlexTouch. While supplies last, Levemir FlexTouch will continue to be provided by pharmacies and distributors. Once the current stock has been used up, no more FlexTouch products will be offered.
Conclusion
Drug shortages can happen for various reasons, such as shortages, stock issues, and problems with manufacturing and safety. The FDA receives the majority of its information on medicine shortages from manufacturers. These two organizations collaborate closely to prevent or lessen the effects of shortages.